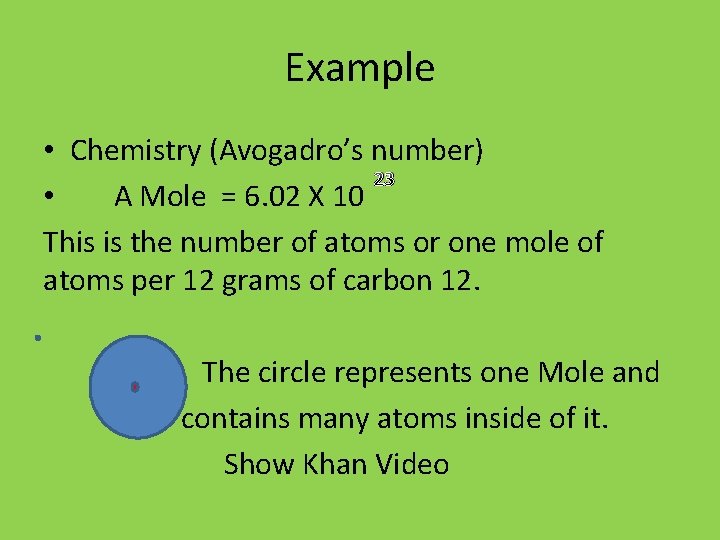
- Avogadro's Number Without Scientific Notation
- Avogadro's Number In Scientific Notation Example
- Avogadro's Number In Scientific Notation Form
Contrary to the beliefs of generations of chemistry students, Avogadro’s number—the number of particles in a unit known as a mole—was not discovered by Amadeo Avogadro (1776-1856). Avogadro was a lawyer who became interested in mathematics and physics, and in 1820 he became the first professor of physics in Italy. Windows 7 repair usb bootable. Avogadro is most famous for his hypothesis that equal volumes of different gases at the same temperature and pressure contain the same number of particles.
Avogadro's Number Without Scientific Notation
The first person to estimate the actual number of particles in a given amount of a substance was Josef Loschmidt, an Austrian high school teacher who later became a professor at the University of Vienna. In 1865 Loschmidt used kinetic molecular theory to estimate the number of particles in one cubic centimeter of gas at standard conditions. This quantity is now known as the Loschmidt constant, and the accepted value of this constant is 2.6867773 x 1025 m-3.
The term “Avogadro’s number” was first used by French physicist Jean Baptiste Perrin. In 1909 Perrin reported an estimate of Avogadro’s number based on his work on Brownian motion—the random movement of microscopic particles suspended in a liquid or gas. In the years since then, a variety of techniques have been used to estimate the magnitude of this fundamental constant.
Accurate determinations of Avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales using the same unit of measurement. This became possible for the first time when American physicist Robert Millikan measured the charge on an electron. The charge on a mole of electrons had been known for some time and is the constant called the Faraday. The best estimate of the value of a Faraday, according to the National Institute of Standards and Technology (NIST), is 96,485.3383 coulombs per mole of electrons. The best estimate of the charge on an electron based on modern experiments is 1.60217653 x 10-19 coulombs per electron. If you divide the charge on a mole of electrons by the charge on a single electron you obtain a value of Avogadro’s number of 6.02214154 x 1023 particles per mole.
Another approach to determining Avogadro’s number starts with careful measurements of the density of an ultrapure sample of a material on the macroscopic scale. The density of this material on the atomic scale is then measured by using x-ray diffraction techniques to determine the number of atoms per unit cell in the crystal and the distance between the equivalent points that define the unit cell (see Physical Review Letters, 1974, 33, 464).
Avogadro's Number In Scientific Notation Example

Avogadro's Number In Scientific Notation Form
Encyclopaedia Britannica states that there are 602,000,000,000,000,000,000,000, or 602 followed by 21 zeroes, atoms in a mole. This number is read aloud as '602 sextillion' and is a reference to Avogadro's number, which is commonly expressed in scientific notation as 6.02 multiplied by 10 to the 23rd power. To calculate the mass of a single atom, first look up the atomic mass of carbon from the periodic table. This number, 12.01, is the mass in grams of one mole of carbon. One mole of carbon is 6.022 x 10 23 atoms of carbon (Avogadro's number). This relation is then used to 'convert' a carbon atom to grams by the ratio. Avogadro's number Avogadro's number is the number of particles in one mole of any substance. Its numerical value is 6.02225 × 1023. One mole of oxygen gas contains 6.02 × 1023molecules of oxygen, while one mole of sodium chloride contains 6.02 × 1023sodium ions and 6.02 × 1023 chloride ions. free download. software arcview full version. The Avogadro's number is equal to (6,022 x 10 raised to 23 particles) and is symbolized in the formulas with the letters L or NA. In addition, it is used to make conversions between grams and atomic mass unit. The unit of measure of the Avogadro's number is the mole (mol-1) but it can also be defined in lb/mol-1 and oz/mol-1.

