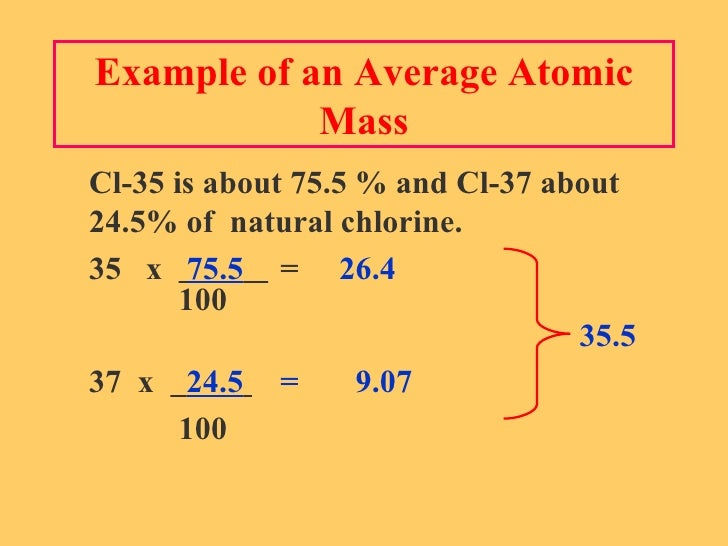
Sample Problem: Calculating Atomic Mass. Use the atomic masses of each of the two isotopes of chlorine along with their percent abundances to calculate the average atomic mass of chlorine. Step 1: List the known and unknown quantities and plan the problem. Chlorine-35: atomic mass = 34.969 amu and% abundance = 75.77%; chlorine-37. For helium, there is approximately one isotope of Helium-3 for every million isotopes of Helium-4; therefore, the average atomic mass is very close to 4 amu (4.002602 amu). Chlorine consists of two major isotopes, one with 18 neutrons (75.77 percent of natural chlorine atoms) and one with 20 neutrons (24.23 percent of natural chlorine atoms). Atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Since 1961 the standard unit of atomic mass has been one-twelfth the mass of an atom of the isotope carbon-12. Atomic weight is measured in atomic mass units (amu), also called daltons. Chlorine (Cl) Atomic Data for Chlorine (Cl) Atomic Number = 17 Atomic Weight = 35.4527 Reference E95: Isotope: Mass: Abundance: Spin: Mag Moment: 35 Cl: 34.968852: 75.77%: 3/2 +0.82187: 37 Cl: 36.965903: 24.23%: 3/2 +0.68412: Cl I Ground State 1s 2 2s 2 2p 6 3s 2 3p 5 2 P.
Click to see full answer
Regarding this, what is the mass of chlorine 35? Exact Masses of the Elements and Isotopic Abundances
| Name | Symbol | Mass |
|---|---|---|
| Chlorine | Cl(35) | 34.968853 |
| Chlorine | Cl(37) | 36.965903 |
| Chromium | Cr(50) | 49.946046 |
| Chromium | Cr(52) | 51.940510 |
What Is Cl Atomic Mass
One may also ask, what is the difference between chlorine 35 and chlorine 37? The number of protons an atom has, also known as the atom's atomic number, determines which element it is. An atom of chlorine-35 contains 18 neutrons (17 protons + 18 neutrons = 35 particles in the nucleus) while an atom of chlorine-37 contains 20 neutrons (17 protons + 20 neutrons = 37 particles in the nucleus).
Correspondingly, what is the meaning of atomic weight of chlorine 35 and 37?
Chlorine has 17 protons in its nucleus and its most common isotope has 18 neutrons. That means that the total mass of a chlorine atom is about 35.5 atomic mass units. Chlorine has 17 protons in its nucleus and its most common isotope has 18 neutrons.
Element Cl Atomic Mass
What Is The Atomic Weight Of Chlorine
Is chlorine 37 radioactive? Free download mp3 white lion till death do us part.
Chlorine Amu
Queentreeblog. There are two stable isotopes, 35Cl (75.77%) and 37Cl (24.23%), giving chlorine a standard atomic weight of 35.45. The mask sound effectncpro. The longest-lived radioactive isotope is 36Cl, which has a half-life of 301,000 years. All other isotopes have half-lives under 1 hour, many less than one second.
